Fri 20 August 2021:
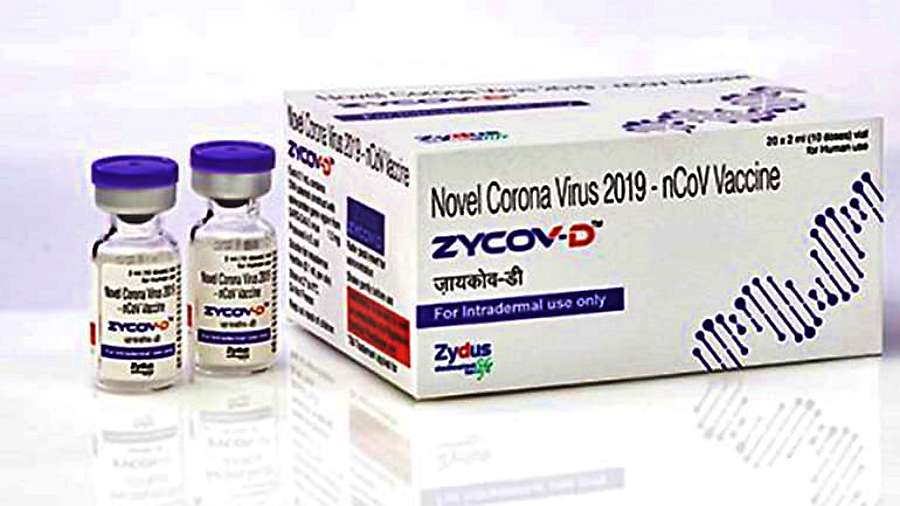
The three-dose COVID-19 DNA vaccine from Zydus Cadila was approved by India’s medicines authority today for emergency use in adults and children aged 12 and up, making it the country’s sixth vaccination to be approved.
The company stated that it intends to produce 100 million to 120 million doses of ZyCoV-D each year and has begun stockpiling the vaccine.
“Zydus Cadila has received approval for emergency use authorization from the DCGI for ZyCoV-D today, the world’s first and India’s indigenously developed DNA based vaccine for COVID-19 to be administered in humans including children and adults 12 years and above,” a statement issued by the federal ministry of science and technology said.
ZyCov-D is developed in partnership with the department of biotechnology and implemented by the Biotechnology Industry Research Assistance Council.
“This three-dose vaccine which when injected produces the spike protein of the SARS-CoV-2 virus and elicits an immune response, which plays a vital role in protection from disease as well as viral clearance. The plug-and-play technology on which the plasmid DNA platform is based can be easily adapted to deal with mutations in the virus, such as those already occurring,” the ministry said.
According to the ministry, interim results from phase-III clinical trials in over 28,000 volunteers showed primary efficacy of 66.6 percent for symptomatic RT-PCR positive cases.
———————————————————————————————————————-
FOLLOW INDEPENDENT PRESS:
TWITTER (CLICK HERE)
https://twitter.com/IpIndependent
FACEBOOK (CLICK HERE)
https://web.facebook.com/ipindependent
Think your friends would be interested? Share this story!