Mon 12 April 2021:
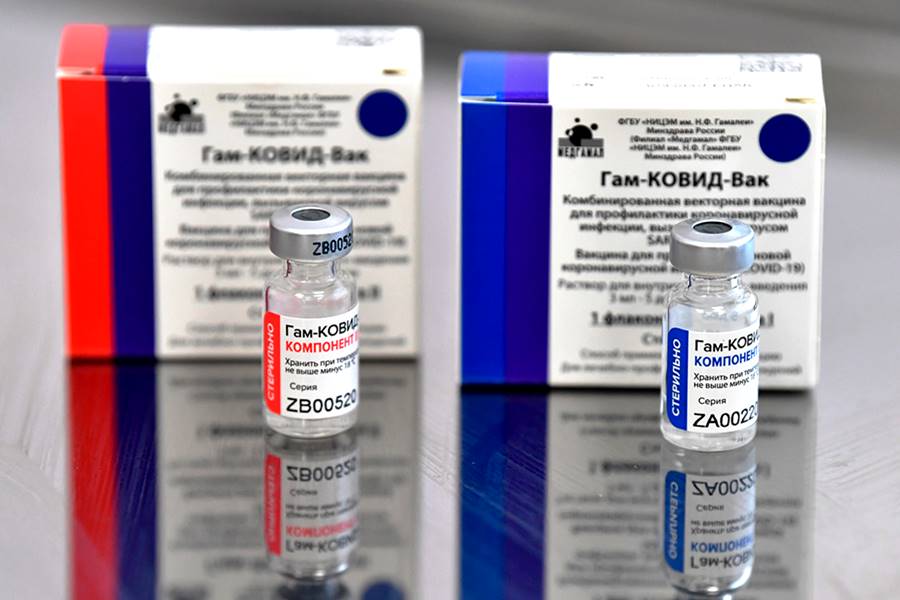
On Monday, India’s Subject Expert Committee of the Central Drugs Standard Control Organisation recommended the Emergency Use Authorization of Russia’s Sputnik V vaccine. If the Drugs Controller General of India accepts this recommendation, it will become the 3rd approved COVID-19 vaccine in India after COVAXIN and COVISHIELD amid surging novel coronavirus cases. To be manufactured in India by Dr. Reddy’s Laboratories, this vaccine has shown the efficacy of 91.6% in the phase 3 trials.
Russian President Vladimir Putin had announced the approval of Sputnik V on August 11, 2020, making it the first vaccine against COVID-19 to be approved in the world. It has been named after the world’s first satellite launched by the Soviet Union.
Developed by the Gamaleya Research Institute of Epidemiology and Microbiology along with the Russian Direct Investment Fund (RDIF), it contains two different human common cold viruses which have been modified to make the SARS-CoV-2 spike protein.
When a vaccinated person comes into contact with COVID-19, the body will have developed an immune response in the form of antibodies to protect it against the virus. Costing less than 10 US dollars for each dose of the vaccine in international markets, the dry form of Sputnik V can be stored at 2-8 degrees celsius.
As per sources, more COVID-19 vaccines such as the Johnson & Johnson vaccine, Novavax vaccine, Zydus Cadila’s vaccine and Bharat Biotech’s Intranasal vaccine are also likely to receive the Centre’s nod in the upcoming months.

FOLLOW INDEPENDENT PRESS:
TWITTER (CLICK HERE)
https://twitter.com/IpIndependent
FACEBOOK (CLICK HERE)
https://web.facebook.com/ipindependent
Think your friends would be interested? Share this story!