Sat 03 July 2021:
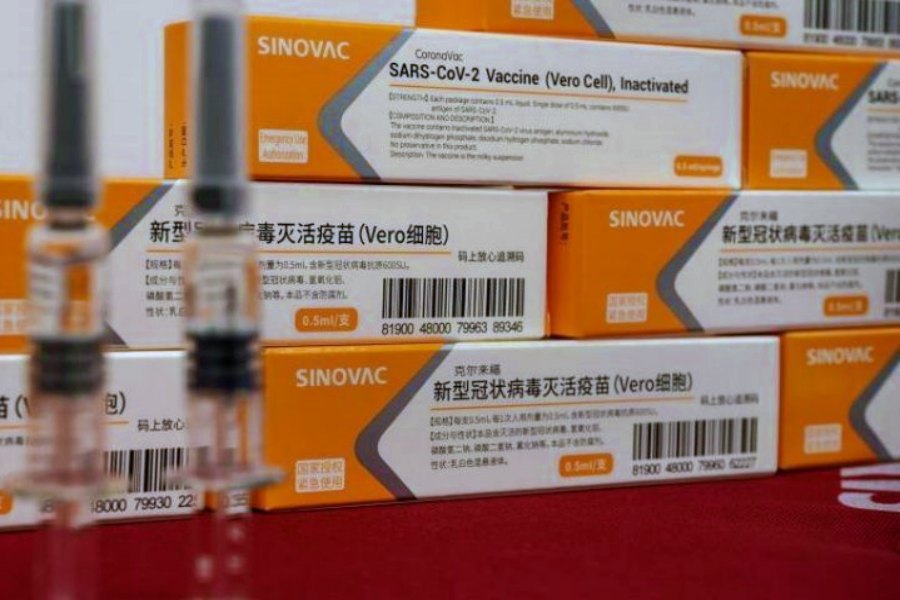
The South African Health Products Regulatory Authority (SAHPRA) on Saturday announced that it had authorized the use of the CoronaVac COVID-19 vaccine developed by Chinese pharmaceutical company Sinovac Biotech.
The latest vaccine approval was based on the “safety, quality and efficacy data” submitted to the regulator between March 22 and June 22, it said in a statement.
“The authorisation is subject to a number of conditions. Specifically, the applicant is required to submit the final results of ongoing clinical studies,” said the authority. “SAHPRA also took account of the World Health Organization Emergency Use Listing report on this vaccine.”
Plenty vaccines to rollout vaccination programme
On the status of the vaccination rollout programme in the country, the deputy director-general at the Department of Health and the man in charge of the government’s Electronic Vaccination Data System (EVDS), Dr Nicholas Crisp said South Africa has received plenty of vaccines to rollout its programme for the next few weeks.
Crisp said in order to scale up the vaccination from 20 000 people currently vaccinating per day to 200 000 and 300 000 people, more vaccination sites are needed to be opened and more people in the remote areas were routinely coming in for vaccination.
“We are already planning for the uptake of the next age of cohorts which is the 40-49, and has seven million people on it. The reason we are targeting age groups, is because there is plenty clinical evidence that age is a major factor more than any factor in keeping people well and out of hospital with severe infections,” Crisp said.
FOLLOW INDEPENDENT PRESS:
TWITTER (CLICK HERE)
https://twitter.com/IpIndependent
FACEBOOK (CLICK HERE)
https://web.facebook.com/ipindependent
Think your friends would be interested? Share this story!