Wed 17 March 2021:
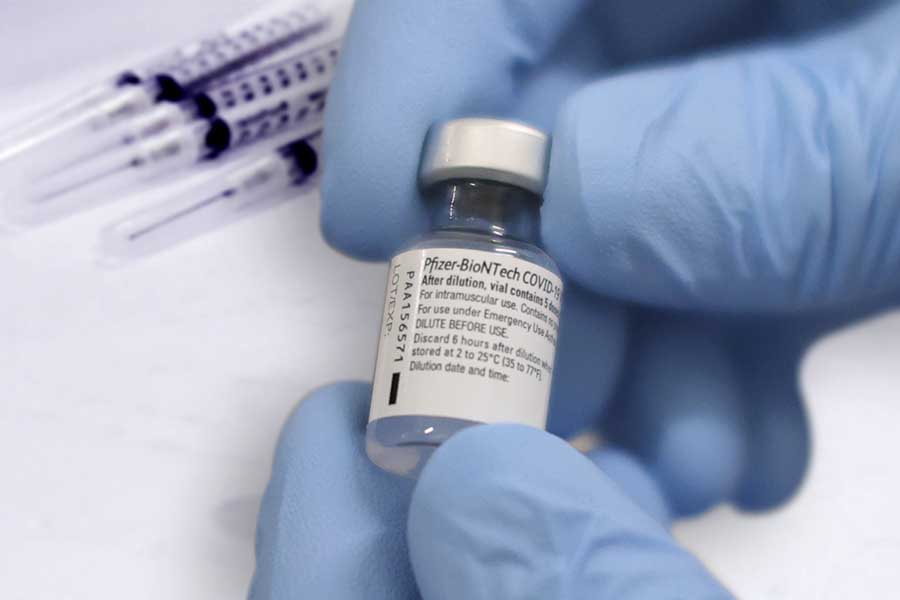
The South African Health Products Regulatory Authority (SAHPRA) has approved the Section 21 application for Pfizer/BioNTech COVID-19 vaccine.
“This approval is subject to conducting a post Section 21 authorisation efficacy and safety surveillance of Comirnaty vaccine in South Africa,” said SAHPRA on Tuesday.
According to SAHPRA, the main purpose of Section 21 is to provide access to medicines on an exceptional basis, where conventional therapies have been ruled out, have failed or are unavailable as marketed products.
“This must always have regard to the safety, efficacy and quality of medicines accessed through Section 21 which are set out in Regulation 29.”
South Africa has ordered 20 million doses of Pfizer/BioNTech vaccines.
Early this month, Health Minister, Dr Zweli Mkhize, said the country was anticipating about 600 000 Pfizer COVID-19 vaccines to land in South Africa before the end of March.
Pfizer/BioNTech is 95% effective, requires two shots and efficacy was consistent across age, gender, race and ethnicity demographics, according to the phase three study.
According to the American multinational pharmaceutical corporation, it is expected to produce up to 1.3 billion doses by the end of 2021.

Meanwhile, more Johnson & Johnson doses of COVID-19 vaccines are expected to arrive this week.
According to the latest data, the number of healthcare workers vaccinated has now risen to 157 286 as of 16 March.
– SAnews.gov.za
FOLLOW INDEPENDENT PRESS:
TWITTER (CLICK HERE)
https://twitter.com/IpIndependent
FACEBOOK (CLICK HERE)
https://web.facebook.com/ipindependent
Think your friends would be interested? Share this story!